Overview¶
- This is a software that can be used to analyze Tn-Seq datasets. It includes various statistical calculations of essentiality of genes or genomic regions (including conditional essentiality between 2 conditions). These methods were developed and tested as a collaboration between the Sassetti lab (UMass) and the Ioerger lab (Texas A&M) [DeJesus2015TRANSIT].
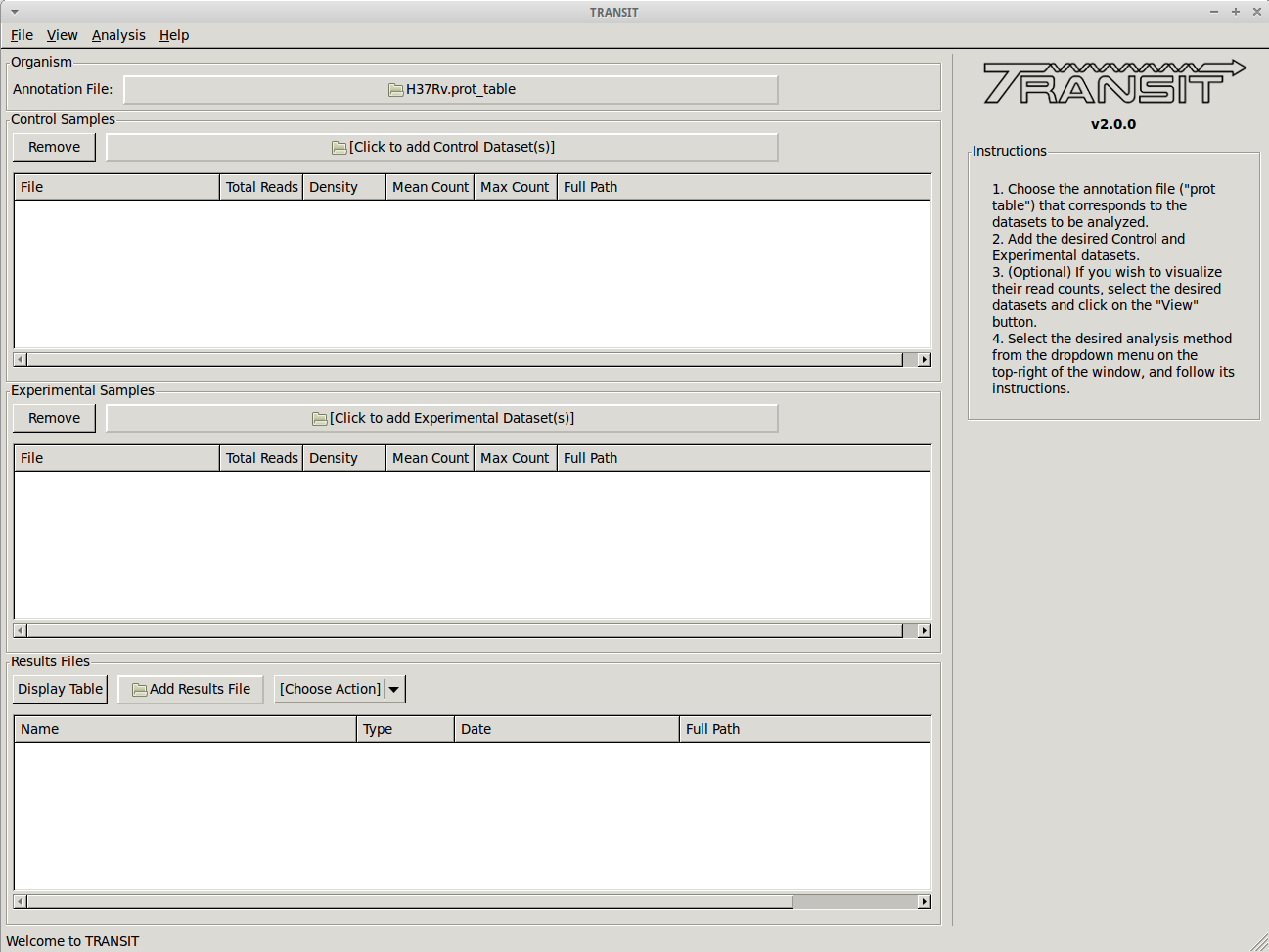
- TRANSIT is capable of analyzing TnSeq libraries constructed with Himar1 or Tn5 datasets.
- TRANSIT assumes you have already done pre-processing of raw sequencing files (.fastq) and extracted read counts into a .wig formatted file. The .wig file should contain the counts at all sites where an insertion could take place (including sites with no reads). For Himar1 datasets this is all TA sites in the genome. For Tn5 datasets this would be all nucleotides in the genome.
- Note that while refer to “read-counts” throughout the documentation, the current Himar1 protocol utilizes internal barcodes that can be used to reduce raw read counts to unique template counts, and this this is the intended input to TRANSIT from Himar1 datasets.
- There are various methods available for pre-processing (converting .fastq files to .wig files). You might have your own scripts (if so, massage the data into .wig format), or you might get the scripts used in the Sassetti lab. For convenience, we are including a separate tool called TPP (Tn-Seq Pre-Processor) with this distribution that encodes the way we process .fastq files in the Ioerger lab. It’s a complicated process with many steps (removing transposon prefixes of reads, mapping into genome, identifying barcodes and reducing read counts to template counts).
- Most of the analysis methods in TRANSIT require an annotation to know the gene coordinates and names. This is the top file input in the GUI window. The annotation has to be in a somewhat non-standard format called a “.prot_table”. If you know what you are doing, it is easy to convert annotations for other organisms into .prot_table format. But for convenience, we are distributing the prot_tables for 3 common versions of the H37Rv genome: H37Rv.prot_table (NC_000962.2, from Stewart Cole), H37RvMA2.prot_table (sequenced version from the Sassetti lab), and H37RvBD.prot_table (sequenced by the Broad Institute). All of these are slightly different, and it is critical that you use the same annotation file as the reference genome sequence used for mapping the reads (during pre-processing).
- There are 2 main types of essentiality analyses: individual, comparative. In individual analysis, the goal is to distinguish essential vs. non-essential in a single growth condition, and to assess the statistical significance of these calls. Two methods for this are the Gumbel method and the HMM. They are computationally distinct. The Gumbel method is looking for significant stretches of TA sites lacking insertions, whereas the HMM looks for regions where the mean read count is locally suppressed or increased. The HMM can detect ‘growth-advantaged’ and ‘growth-defect’ regions. The HMM is also a bit more robust on low-density datasets (insertion density 20-30%). But both methods have their merits and are complementary. For comparative analysis, TRANSIT uses ‘re-sampling’, which is analogous to a permutation test, to determine if the sum of read counts differs significantly between two conditions. Hence this can be used to identify conditionally essential regions and quantify the statistical significance.
- TRANSIT has been designed to handle multiple replicates. If you have two or more replicate dataset of the same library selected in the same condition, you can provide them, and more of the computational methods will do something reasonable with them.
- For those methods that generate p-values, we often also calculate adjusted p-value (or ‘q-values’) which are corrected for multiple tests typically the Benjamini-Hochberg procedure. A typical threshold for significance would be q<0.05 (not p<0.05).
- It is important to understand the GUI model that TRANSIT uses It allows you to load up datasets (.wig files), select them, choose an analysis method, set parameters, and start the computation. It will generate output files in your local directory with the results. These files can then be loaded into the interface and browser with custom displays and graphs. The interface has 3 main windows or sections: ‘Control Samples’, ‘Experimental Samples’, ‘Results Files.’ The first two are for loading input files (‘Control Samples’ would be like replicate datasets from a reference condition, like in vitro, rich media, etc.; ‘Experimental Samples’ would be where you would load replicates for a comparative conditions, like in vivo, or minimal media, or low-iron, etc.) The ‘Results Files’ section is initially empty, but after a computation finishes, it will automatically be populated with the corresponding output file. See the ‘Tutorial’ section below in this documentation for an illustration of the overall process for a typical work-flow.
- TRANSIT incorporates many interesting ways of looking at your data.
- Track view shows you a visual representation of the read counts at each site at a locus of interest (for selected datasets) somewhat like IGV.
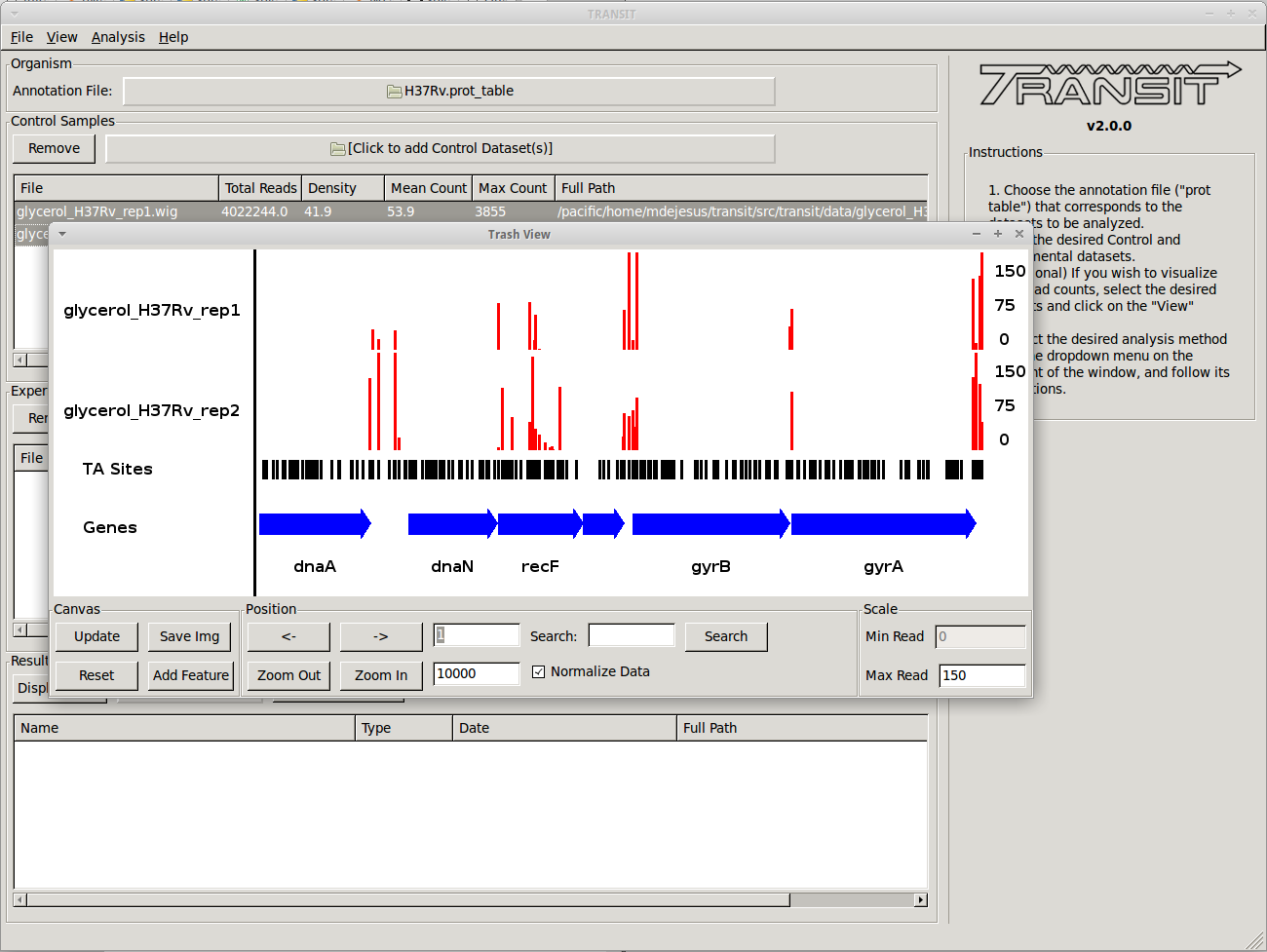
- Scatter plots can show the correlation of counts between 2 datasets.
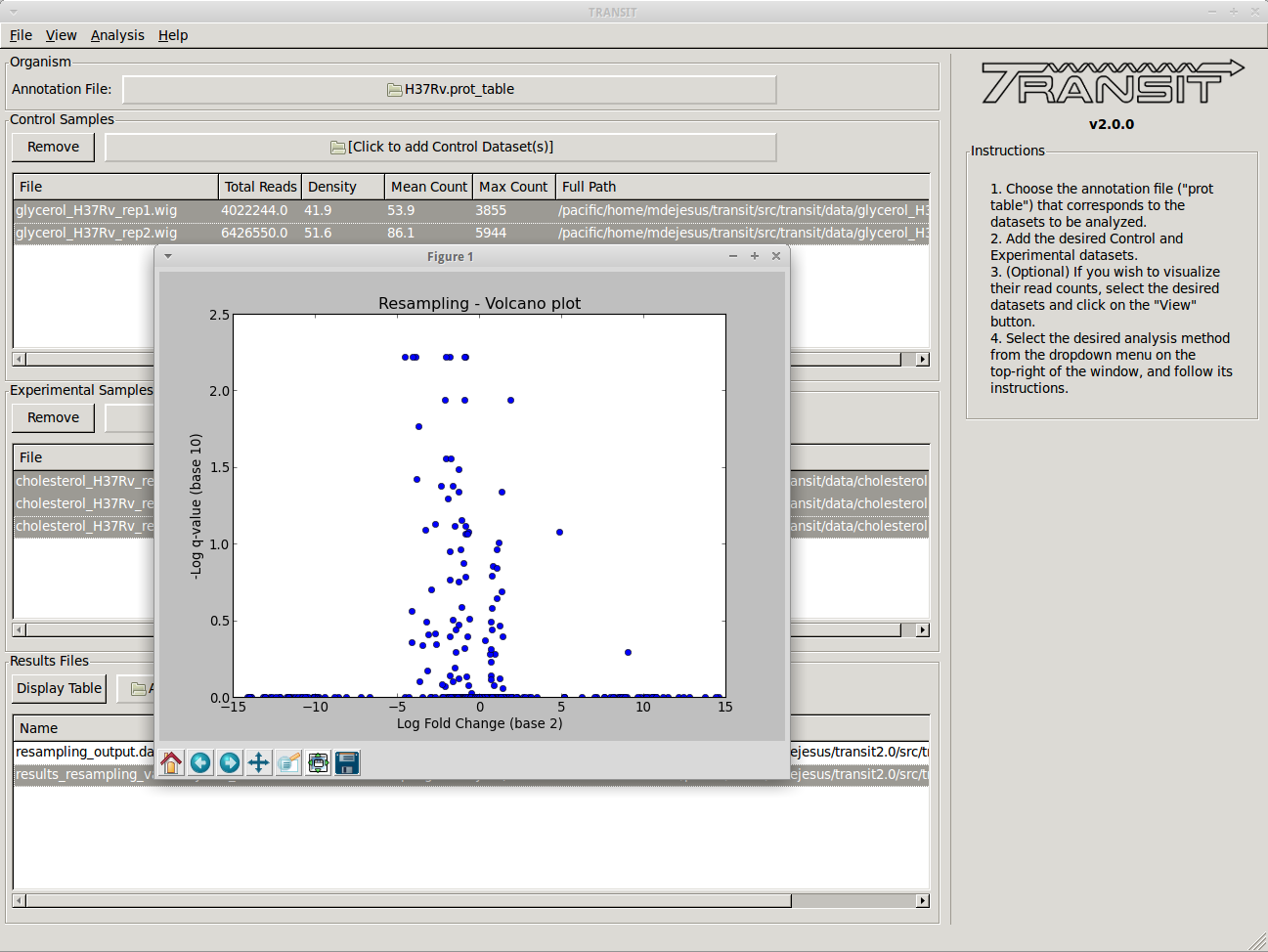
- Most of the methods take a few minutes to run. (it depends on parameters, CPU clock speed, etc., but the point is, a) these calculations are complex and not instaneous, but b) we have tried to implement it so that they don’t take hours)
- Note: in this version of TRANSIT, most of the methods are oriented toward gene-level analysis. There are methods for analyzing essentiality of arbitrary genomic regions (e.g. sliding windows, HMMs…). We plan to incorporate some of these in future versions.
Tn5 Datasets¶
Transit can now process and analyze Tn5 datasets This is a different transposon than Himar1. The major difference is Tn5 can insert at any site in the genome, and is not restricted to TA dinucleotides (and saturation is typically much lower). This affects the statistical analyses (which were originally designed for Himar1 and can’t directly be applied to Tn5). Therefore, Resampling was extended to handle Tn5 for comparative analysis, and Tn5Gaps is a new statistical model for identifying essential genes in single Tn5 datasets. Amplification of Tn5 libraries uses different primers, and this affects the pre-processing by TPP. But TPP has be modified to recognize the primer sequence for the most widely used protocol for Tn5. Furthermore, TPP now has an option for users to define their own primer sequences, if they use a different sample prep protocol.
Developers¶
| Name | Time Active | Contact Information |
|---|---|---|
| Michael A. DeJesus | 2015-Present | http://mad-lab.org |
| Thomas R. Ioerger | 2015-Present | http://faculty.cs.tamu.edu/ioerger/ |
| Chaitra Ambadipudi | 2015 | |
| Eric Nelson | 2016 |
References¶
If you use TRANSIT, please cite the following reference:
| [DeJesus2015TRANSIT] | DeJesus, M.A., Ambadipudi, C., Baker, R., Sassetti, C., and Ioerger, T.R. (2015). TRANSIT - a Software Tool for Himar1 TnSeq Analysis. PLOS Computational Biology, 11(10):e1004401 |
Development of TRANSIT is funded by the National Institutes of Health (www.nih.gov/) grant U19 AI107774.
Other references, including methods utilized by TRANSIT: